New research out of the Harvard School of Dental Medicine (HSDM) sheds light on a largely underexplored frontier of developmental biology – how mechanical stimuli control growth and patterning in embryonic development. The findings, reported in Science Advances, could have implications for preventing or treating neural tube birth defects in humans.
Previous studies primarily focused on understanding the complex networks of biochemical signaling pathways that control the development of higher vertebrate embryos and had yet to fully consider the interplay of biophysical stimuli that cells receive.
Mechanical information such as stress, strain, and fluid flow, which are generated by gravity, cell movement, and cell-cell or cell-extracellular matrix interactions are constantly collected by embryonic cells. Yet a functional understanding of mechanotransduction remains limited and it is not clear how biophysical and biochemical stimuli are integrated.
Looking at the formation of the notochord, a central organizer that determines the dorsal (back) -ventral (belly) (DV) body plan in early vertebrate development, the researchers found that biophysical stimuli generated during embryonic morphogenesis direct critical growth and patterning of midline structures, including the neural tube (NT), the embryonic precursor of the central nervous system (CNS).
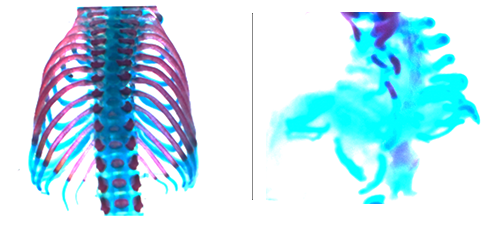
At the molecular levels, FoxA2 and Shh are known to be ventrally located key regulators of notochord formation and NT DV patterning, but it has been unknown how the regionally restricted expression of FoxA2 and Shh is determined. The findings identify a gradient of mechanical stress and tissue stiffness in the notochord and NT generated during morphogenesis. The highest mechanical stress in the notochord and ventral NT acts as a crucial initial event that leads to activation of the Yap transcription factor in the notochord and the ventral-most NT structure, the floor plate (FP). Activated Yap directly drives FoxA2 and Shh expression.
“Our findings uncover a novel and fundamentally important function of Yap in integrating mechanical stimuli with biochemical signaling activation in establishing signaling centers that guide embryonic growth and patterning,” said Dr. Yingzi Yang, the leading principal investigator (PI) of the project. Yang is professor of developmental biology and associate dean for research at HSDM. “We expect that this report will have broad interest and appeal to basic and translational scientists working in the areas of developmental biology, human genetic diseases, neural diseases, stem cell biology, mechanotransduction and Hippo signaling.”
By providing new insights into notochord and NT development, these findings present Yap as potential targets for preventing or treating NT defects (NTDs). NTDs are one of the most common birth defects in humans and can result in malformation of the gut, neural tube, vertebrae, and cranial region. Despite limited interventions of surgery corrections and folic acid supplementation during pregnancy, a significant portion of NTDs remain unpreventable and/or difficult to treat.
Importantly, these new findings about the critical feed-forward roles of Yap in controlling FoxA2 and Shh expression unify the research team’s previous work. They demonstrated that direct activation of Shh expression by Yap in a completely different and FoxA2- independent context: the disease of heterotopic ossification, which is caused by abnormal osteoblast differentiation in soft tissues in adult human beings.
Drs. Caiqi Cheng and Qian Cong are co-first authors of the paper. Cheng was a visiting student in the Yang Lab and Cong is an instructor in the Yang Lab at HSDM. This work is supported by NIH grants R01DE025866, R01AR070877 and R01CA222571, as well as the DoD grant PR201987 to Yang.